
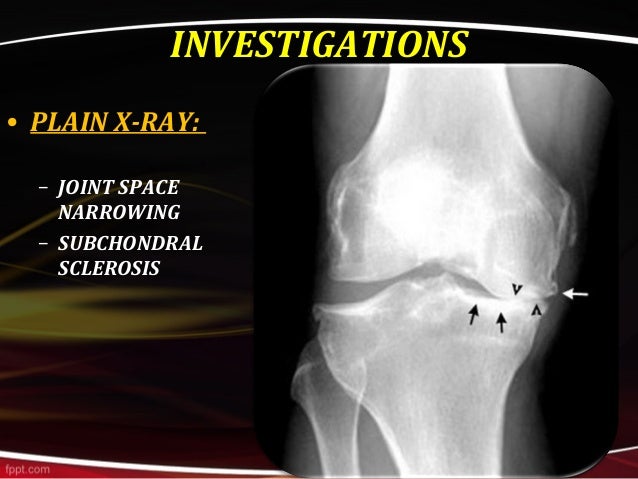
20 Accumulating evidences suggest that mTORC1 is essential for maintenance of chondrocyte metabolic homeostasis and its activation in articular chondrocyte plays a vital role in OA development. mTORC1 is a sensitive target of rapamycin and is suppressed by the functional complex, tuberous sclerosis complex 1/2 (TSC1/2). 18, 19 mTOR forms two distinct functional complexes, mTOR complex 1 (mTORC1) and mTORC2. Mechanistic target of rapamycin (mTOR) is a highly conserved Ser/Thr protein kinase that functions as a master regulator of cell growth, proliferation, survival, and metabolism in response to nutrients, growth factors, and stresses. 17 However, the mechanism by which aberrant subchondral bone formation is induced and the interplay between increased subchondral bone turnover and articular cartilage degeneration during OA remains unclear. Thus, changes in the subchondral bone have been suggested to predict the severity of cartilage damage in OA. In a situation of instability, such as occurring with ligament injury, 14 excessive body weight, 15 or weakening muscles related to aging, 16 the mechanical loading on weight-bearing joints is dramatically increased, and changes in the subchondral bone microarchitecture may precede articular cartilage damage. 12 Indeed, homeostasis and integrity of articular cartilage rely on its biochemical and biomechanical interplay with subchondral bone and other joint tissues, 13 and treatment targeting articular cartilage alone in OA has proven to be insufficient to halt disease progression. Relative to the slower turnover rate of articular cartilage, subchondral bone undergoes more rapid modeling and remodeling in response to changes of the mechanical environment.
1, 10, 11 Subchondral bone provides mechanical support for the overlying articular cartilage during the movement of joints and absorbs most of the mechanical force transmitted by diarthrodial joints. Recently, increasing evidences indicate that articular cartilage degeneration is related to aberrant subchondral bone turnover. 5 Despite the identified risk factors, which include genetic predisposition, 6 mechanical abnormality, 7 age, 8 and obesity, 9 the exact pathogenesis of OA remains unclear. 4 Indeed, the development of OA disease affects all joint tissues, being characterized by progressive degeneration of articular cartilage, vascular invasion of the articular surface, subchondral bone remodeling, osteophyte formation, and synovial inflammation. However, no effective disease-modifying treatment for OA is currently available until the disease reaches the end stage, necessitating joint replacement. Osteoarthritis (OA) is a highly prevalent and degenerative joint disorder which mainly affects the weight-bearing joints such as hips and knees, and is the leading cause of physical disability, 1, 2, 3 presenting an enormous clinical and financial burden. Pharmaceutical inhibition of the pathway presents a promising therapeutic approach for OA treatment. Altogether, these findings demonstrate that mTORC1 activation in subchondral preosteoblasts is not sufficient to induce OA, but can induce aberrant subchondral bone formation and secrete of Cxcl12 to accelerate disease progression following surgical destabilization of the joint. A Cxcl12-neutralizing antibody reduced cartilage degeneration and alleviated OA in mice. Mechanistically, mTORC1 activation promoted preosteoblast expansion and Cxcl12 secretion, which induced subchondral bone remodeling and cartilage degeneration during OA. In contrast, inhibition of mTORC1 in preosteoblasts by disruption of Raptor (mTORC1-specific component) reduced subchondral bone formation and cartilage degeneration, and attenuated post-traumatic OA in mice. Constitutive activation of mTORC1 in preosteoblasts by deletion of the mTORC1 upstream inhibitor, tuberous sclerosis 1, induced aberrant subchondral bone formation, and sclerosis with little-to-no effects on articular cartilage integrity, but accelerated post-traumatic OA development in mice. Here, we show that the mechanistic target of rapamycin complex 1 (mTORC1) pathway is activated in subchondral bone preosteoblasts (Osterix+) from OA patients and mice. However, how subchondral bone formation is activated and the mechanism by which increased subchondral bone turnover promotes cartilage degeneration during OA remains unclear. Increasing evidences show that aberrant subchondral bone remodeling plays an important role in the development of osteoarthritis (OA).


 0 kommentar(er)
0 kommentar(er)
